Ozempic pen – How much does it cost and where can I buy it?
0₫
Ozempic pen 0.25 0.5 1mg Semaglutide how much doses it cost and where can i buy it? What Are Side Effects of Ozempic pen? Therapeutic indications Pen?
Contact 0969870429
Name of the medicinal product
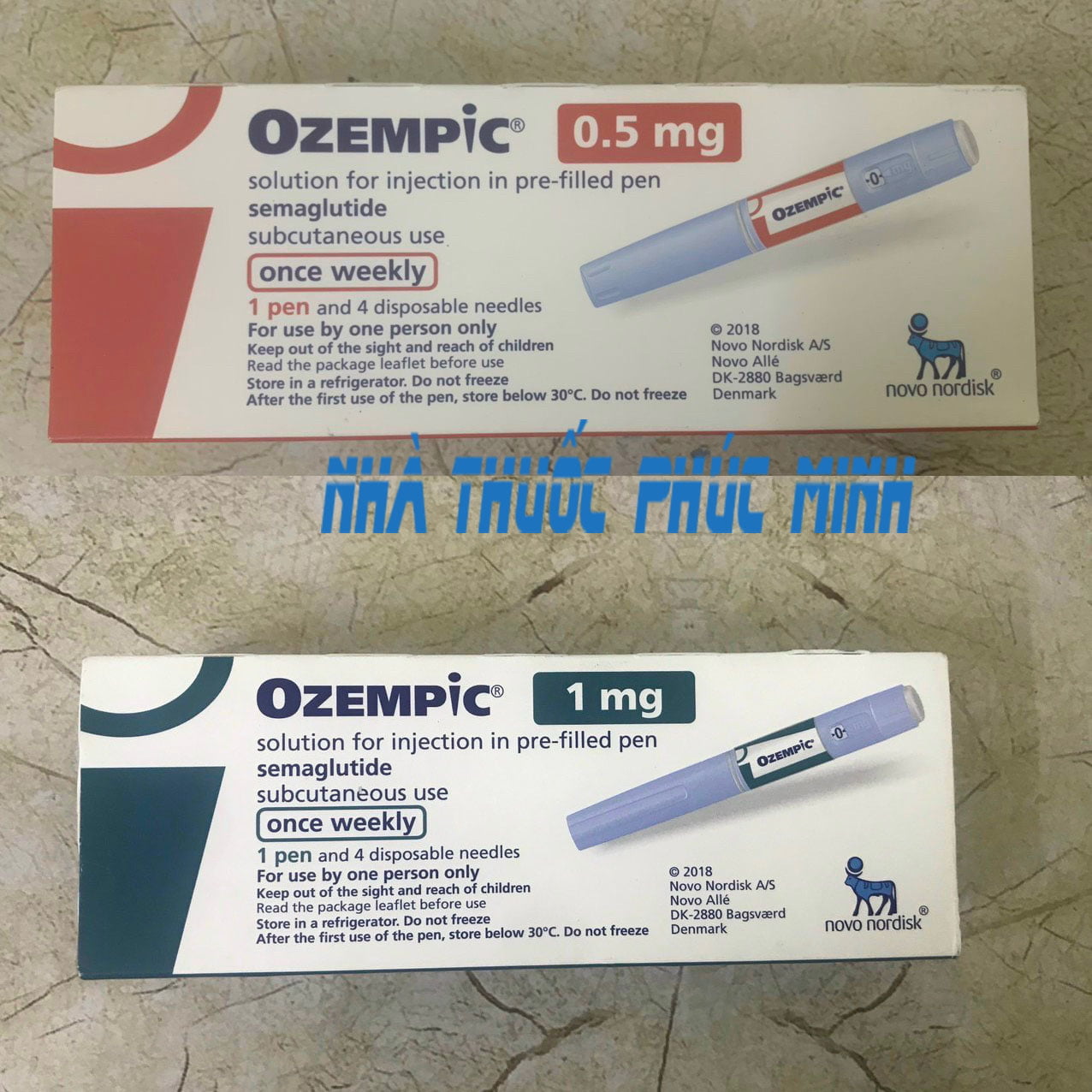
Ozempic 0.25 mg solution for injection in pre-filled pen
Ozempic 0.5 mg solution for injection in pre-filled pen
Ozempic 1 mg solution for injection in pre-filled pen.
Qualitative and quantitative composition
One ml of solution contains 1.34 mg of semaglutide*. One pre-filled pen contains 2 mg semaglutide* in 1.5 ml solution. Each dose contains 0.25 mg of semaglutide in 0.19 ml solution.
Human glucagon-like peptide-1 (GLP-1) analogue produced in Saccharomyces cerevisiae cells by recombinant DNA technology.
Therapeutic indications Ozempic
Ozempic is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
• as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
• in addition to other medicinal products for the treatment of diabetes.
Posology and method of administration Ozempic
Posology
The starting dose is 0.25 mg semaglutide once weekly. After 4 weeks the dose should be increased to 0.5 mg once weekly. After at least 4 weeks with a dose of 0.5 mg once weekly, the dose can be increased to 1 mg once weekly to further improve glycaemic control. After at least 4 weeks with a dose of 1 mg once weekly, the dose can be increased to 2 mg once weekly to further improve glycaemic control.
Semaglutide 0.25 mg is not a maintenance dose. Weekly doses higher than 2 mg are not recommended.
When Ozempic is added to existing metformin and/or thiazolidinedione therapy or to a sodium-glucose cotransporter 2 (SGLT2) inhibitor, the current dose of metformin and/or thiazolidinedione or SGLT2 inhibitor can be continued unchanged.
When Ozempic is added to existing therapy of sulfonylurea or insulin, a reduction in the dose of sulfonylurea or insulin should be considered to reduce the risk of hypoglycaemia.
Self-monitoring of blood glucose is not needed in order to adjust the dose of Ozempic. Blood glucose self-monitoring is necessary to adjust the dose of sulfonylurea and insulin, particularly when Ozempic is started and insulin is reduced. A stepwise approach to insulin reduction is recommended.
Missed dose
If a dose is missed, it should be administered as soon as possible and within 5 days after the missed dose. If more than 5 days have passed, the missed dose should be skipped, and the next dose should be administered on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
Changing the dosing day
The day of weekly administration can be changed if necessary, as long as the time between two doses is at least 3 days (>72 hours). After selecting a new dosing day, once-weekly dosing should be continued.
Special populations
Elderly
No dose adjustment is required based on age. Therapeutic experience in patients ≥75 years of age is limited.
Renal impairment
No dose adjustment is required for patients with mild, moderate or severe renal impairment. Experience with the use of semaglutide in patients with severe renal impairment is limited. Semaglutide is not recommended for use in patients with end-stage renal disease.
Hepatic impairment
No dose adjustment is required for patients with hepatic impairment. Experience with the use of Semaglutide in patients with severe hepatic impairment is limited. Caution should be exercised when treating these patients with semaglutide.
Paediatric population
The safety and efficacy of semaglutide in children and adolescents below 18 years have not yet been established. No data are available.
Method of administration
Subcutaneous use.
Ozempic is to be injected subcutaneously in the abdomen, in the thigh or in the upper arm. The injection site can be changed without dose adjustment. Ozempic should not be administered intravenously or intramuscularly.
Ozempic is to be administered once weekly at any time of the day, with or without meals.
Contraindications
Hypersensitivity to the active substance or to any of the excipients list.
Special warnings and precautions for use Ozempic
Traceability
In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
General
Semaglutide should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis. Semaglutide is not a substitute for insulin. Diabetic ketoacidosis has been reported in insulin-dependent patients whom had rapid discontinuation or dose reduction of insulin when treatment with a GLP-1 receptor agonist is started.
There is no experience in patients with congestive heart failure NYHA class IV and semaglutide is therefore not recommended in these patients.
Gastrointestinal effects
Use of GLP-1 receptor agonists may be associated with gastrointestinal adverse reactions. This should be considered when treating patients, with impaired renal function as nausea, vomiting, and diarrhoea may cause dehydration which could cause a deterioration of renal function.
Acute pancreatitis
Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, semaglutide should be discontinued; if confirmed, semaglutide should not be restarted. Caution should be exercised in patients with a history of pancreatitis.
Hypoglycaemia
Patients treated with semaglutide in combination with a sulfonylurea or insulin may have an increased risk of hypoglycaemia. The risk of hypoglycaemia can be lowered by reducing the dose of sulfonylurea or insulin when initiating treatment with semaglutide.
Diabetic retinopathy
In patients with diabetic retinopathy treated with insulin and semaglutide, an increased risk of developing diabetic retinopathy complications has been observed. Caution should be exercised when using semaglutide in patients with diabetic retinopathy treated with insulin. These patients should be monitored closely and treated according to clinical guidelines. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy, but other mechanisms cannot be excluded.
There is no experience with semaglutide 2 mg in patients with type 2 diabetes with uncontrolled or potentially unstable diabetic retinopathy and semaglutide 2 mg is therefore not recommended in these patients.
Interaction with other medicinal products and other forms of interaction
Semaglutide delays gastric emptying and has the potential to impact the rate of absorption of concomitantly administered oral medicinal products. Semaglutide should be used with caution in patients receiving oral medicinal products that require rapid gastrointestinal absorption.
Paracetamol
Semaglutide delays the rate of gastric emptying as assessed by paracetamol pharmacokinetics during a standardised meal test. Paracetamol AUC0-60min and Cmax were decreased by 27% and 23%, respectively, following concomitant use of semaglutide 1 mg. The total paracetamol exposure (AUC0-5h) was not affected. No clinically relevant effect on the rate of gastric emptying was observed with semaglutide 2.4 mg, following 20 weeks of administration of semaglutide, probably due to a tolerance effect. No dose adjustment of paracetamol is necessary when administered with semaglutide.
Oral contraceptives
Semaglutide is not anticipated to decrease the effect of oral contraceptives as semaglutide did not change the overall exposure of ethinylestradiol and levonorgestrel to a clinically relevant degree when an oral contraceptive combination medicinal product (0.03 mg ethinylestradiol/0.15 mg levonorgestrel) was co-administered with semaglutide. Exposure of ethinylestradiol was not affected; an increase of 20% was observed for levonorgestrel exposure at steady state. Cmax was not affected for any of the compounds.
Atorvastatin
Semaglutide did not change the overall exposure of atorvastatin following a single dose administration of atorvastatin (40 mg). Atorvastatin Cmax was decreased by 38%. This was assessed not to be clinically relevant.
Digoxin
Semaglutide did not change the overall exposure or Cmax of digoxin following a single dose of digoxin (0.5 mg).
Metformin
Semaglutide did not change the overall exposure or Cmax of metformin following dosing of 500 mg twice daily over 3.5 days.
Warfarin
Semaglutide did not change the overall exposure or Cmax of R- and S-warfarin following a single dose of warfarin (25 mg), and the pharmacodynamic effects of warfarin as measured by the international normalised ratio (INR) were not affected in a clinically relevant manner. However, upon initiation of semaglutide treatment in patients on warfarin or other coumarin derivatives, frequent monitoring of INR is recommended.
Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential are recommended to use contraception when treated with semaglutide.
Pregnancy
Studies in animals have shown reproductive toxicity. There are limited data from the use of semaglutide in pregnant women. Therefore, semaglutide should not be used during pregnancy. If a patient wishes to become pregnant, or pregnancy occurs, semaglutide should be discontinued. Semaglutide should be discontinued at least 2 months before a planned pregnancy due to the long half-life.
Breast-feeding
In lactating rats, semaglutide was excreted in milk. As a risk to a breast-fed child cannot be excluded, semaglutide should not be used during breast-feeding.
Fertility
The effect of semaglutide on fertility in humans is unknown. Semaglutide did not affect male fertility in rats. In female rats, an increase in oestrous length and a small reduction in number of ovulations were observed at doses associated with maternal body weight loss.
Effects on ability to drive and use machines
Semaglutide has no or negligible influence on the ability to drive or use machines. When it is used in combination with a sulfonylurea or insulin, patients should be advised to take precautions to avoid hypoglycaemia while driving and using machines.
What Are Side Effects of Ozempic?
Ozempic may cause serious side effects including:
- a lump in the neck,
- difficulty swallowing,
- cough,
- shortness of breath,
- difficulty breathing,
- upper abdominal pain,
- nausea,
- vomiting,
- blurred vision,
- spots or dark strings floating in your vision,
- fluctuating vision,
- vision loss,
- dark or empty areas in your vision,
- shakiness,
- nervousness,
- anxiety,
- sweating,
- chills,
- clamminess,
- irritability,
- impatience,
- confusion,
- fast heartrate,
- lightheadedness,
- dizziness,
- hunger,
- decreased urination,
- swelling in your legs, ankles, or feet,
- fatigue,
- rash,
- itching, and
- shock
Get medical help right away, if you have any of the symptoms listed above.
Common side effects of Ozempic include:
- nausea,
- vomiting,
- diarrhea,
- abdominal pain and constipation.
How much does an ozempic pen cost?
Contact 0969870429 for a quote.
Where to buy ozempic injection pen?
Contact 0969870429.
Hà Nội: 20 Cự Lộc, Thanh Xuân.
Hồ Chí Minh: 1646 Võ Văn Kiệt, quận 8.
References
Sản phẩm tương tự
Chưa phân loại
Chưa phân loại
Chưa phân loại
Chưa phân loại